

Ch20; Heat and the First Law of Thermodynamics
Work and Heat
in Thermodynamic Processes
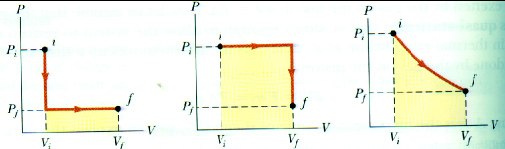
Work Done by a Gas
When a gas expands it does work on its surroundings. That work is equal to the area under the curve on a P-V diagram which describes that expansion.
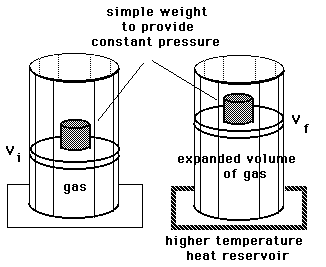
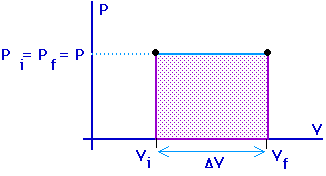
Isobaric process:Process at constant pressure:
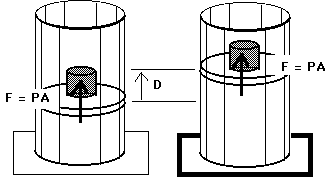
W = F D
W = ( F / A ) ( A D )
W = P ( A D )
W = P (
V)
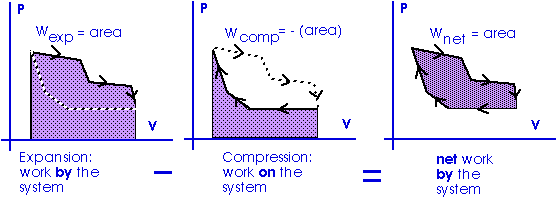
Work = area under the curve on a P-V diagram.
Work = area under the curve on a P-V diagram -- even if the process is not isobaric.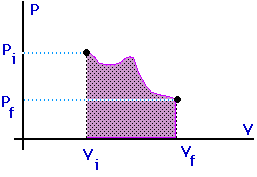
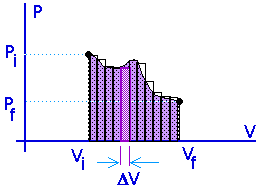
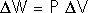
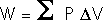
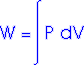
Isometric processes:Isochoric:
Constant volume:
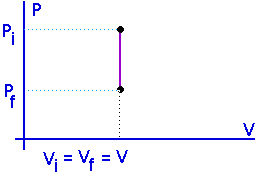
No work is done in an isometric process.
Isothermal process:Process at constant temperature:
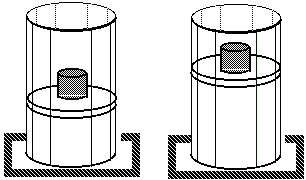
We can keep the temperature constant by having the system in contact with a heat reservoir.

Adiabatic process:Process with zero heat flow:
Insulated:
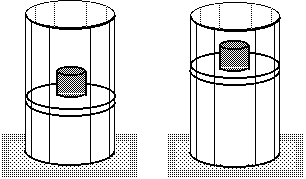
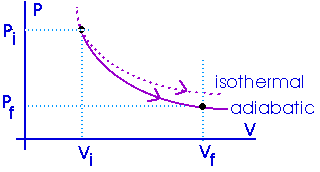
The amount of work done will be less than for an isothermal process between the same two volumes. Notice that an adiabat is steeper than an isotherm.
Example: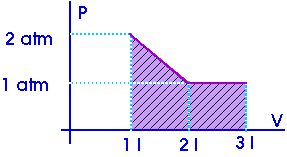
W = 2.5 atm-l
Now, what is an "atmosphere-liter"? It is a pressure (atm) multiplied by a volume (liter) so it must be some sort of work or energy. Now, this is just a units conversion question,
W = 2.5 atm-l [ 0.001 m3 / 1 l ] [ 1.013 x 105 Pa / 1 atm ] W = 253 Pa-m3 [ (N/m2) / Pa ]
W = 253 N-m
W = 253 J
Return to Ch20 ToC
(c) Doug Davis, 2002; all rights reserved