

Kinetic Theory of Gases
Specific Heat of an Ideal Gas We have written
Q = c m T
for heat added to liquids and solids. We did not ask for additional information. However, we do need additional information when we talk about heat added to a gas. Remember the First Law of Thermodynamics,
Q = W + U
and the work W done by the gas depends -- very strongly! -- on how the process is carried out. That means the heat Q will also depend upon how the process is carried out.
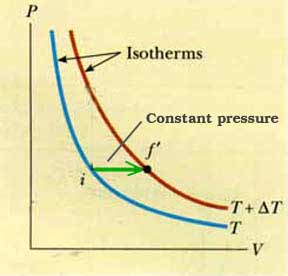
Consider these two isotherms at temperatures T and T +
T. The "area under the curve" is drastically different for different paths. So the amount of heat necessary to change the temperature by an amount
T depends upon the process (or path) taken. In particular, we will look at two common processes:
Q = n CV T (for constant volume)
Q = n CP
T (for constant pressure)
n is the number of moles and CV is the molar specific heat at constant volume and CP is the molar specific heat at constant pressure.
T, of course, is the change in temperature.
On the previous web page we found
that is, the kinetic energy of a gas molecule is directly related to the temperature. For a monatomic gas, the total internal energy U must be the sum of the translational kinetic energy of all of the gas molecules (or atoms). We can write this as
U = N [ KE ] = N [(1/2) m <v2>] U = (3/2) N k T = (3/2) n R T
If we now add heat at constant volume -- so that no work is done -- all of that heat Q does only one thing. It increases the internal energy U,
Q = U = (3/2) n R
T
Q = n CV
T = (3/2) n R
T
n CV = (3/2) n R
CV = (3/2) R
That means that
CV = (3/2) R = 12.5 J/mol-K for all monatomic gases -- primarily the noble gases, He, Ne, Ag, Xe, etc. And this is in good agreement with what we find experimentally (as it ought to be!).
Now, look at a constant pressure process,
Q = n CP
T
W = P
V
U = Q - W
U = n CP
T - P
V
For the same change in temperature,
T, the change in the internal energy
U must be the same for both processes,
n CV T = n CP
T - P
V
CV = CP - P
V/(n
T)
CV = CP - R
CP - CV = R
CP = (5/2) R
It is often useful to describe, calculate, predict, or measure the ratio of these two specific heats,
These values for CV, CP, and
are in good agreement with experimental results.
Return to Ch 21 ToC (c) Doug Davis, 2002; all rights reserved