
PHY1161C
Key #4
December 6, 2000

PHY1161C
Key #4
December 6, 2000
Possibly useful information:
c = 3.00 x 108 m/s ![]() h
= 6.626 x 10-34 Js = 4.14 x 10-15 eV s
h
= 6.626 x 10-34 Js = 4.14 x 10-15 eV s
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
E = m c2 ![]() E
= h f
E
= h f
KEmax = h f - Wo ![]()
![]() = h/p = h / mv
= h/p = h / mv
(![]() x)
(
x)
(![]() px)
Å h
px)
Å h ![]() for
SHO, Eo = h f / 2
for
SHO, Eo = h f / 2
![]() R = 1.097 x 107 m - 1
R = 1.097 x 107 m - 1 ![]()
![]() E
= Ei - Ef = h f
E
= Ei - Ef = h f
![]() ,
, ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Subshells: l = 0, 1, 2, 3, . . .; ![]() s,
p, d, f, g, h, . . .
s,
p, d, f, g, h, . . .
1 u = 931 MeV/c2
--->
+
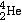
Q = ( mP - mD - ma) c2
--- >
+ b- +

Q = ( mP - mD ) c2
--- >
+ b+ + n
Q = ( mP - mD - 2 mb ) c2
N = No e -t
T1/2 = 0.693/
| Return to 1161 Calendar |
1. [25 pts] (similar to
27.2) A flashbulb goes off at the origins as the origins of
our usual reference frames A and B coincide. Frames A and B have a
relative speed of 0.8 c along their x-axes. A wavefront travels along
the x-axis as observed in B.
a) [ 6 pts] What time is recorded in B as this
wavefront reaches xB = 1,000 m?
b) {14 pts] Use these values of xB and
tB to calculate the coordinates xA and
tA that describe this event in A.
c) { 5 pts] Then use these values to calculate the speed
of light in reference frame A ( vlight =
xA/tA).

To find time tB we just use the definition of velocity
Of course, we also have

Now we can apply the Lorentz Transformations to find xA and tA,

Now we can find the velocity in the A-frame and determine if the Lorentz Transformations work. Is it, indeed, true that the velocity of light will be the same in both frames? Let’s see, . . .
And, indeed, this is the speed of
light!
2. [15 pts] (similar to 27.49) A spaceship
travels away from Earth at a velocity of
2.5 x 108 m/s. A projectile is shot forward from
the spaceship at 1.5 x 108 m/s relative to the spaceship.
What is the velocity of the projectile relative to Earth?

This provides a more direct application of our velocity transformation equation,
with


3. [10 pts] (28.15) What is the energy of the
photons in the visible region of the electromagnetic spectrum? (Use
![]() = 550 nm as an average value)?
= 550 nm as an average value)?
4. [15 pts] (28.41) What is the wavelength of
an electron moving at 1.5 x 108 m/s (v =
0.5 c)?
This wavelength is larger about a nuclear diameter
and is a reasonable fraction of typical atomic spacing.
5. [15 pts] (29.1) Calculate the first
wavelength of the Balmer series for hydrogen and tell what color it
is.
Balmer Series for Hydrogen:
For n = 3 we have
and this is red light
6. [20 pts] (29.16) In state “A” the
energy of an atom is - 3.45 eV and in state “B” its
energy is - 5.67 eV.
a) [7 pts] What is the energy of the photon emitted in
the transition from “A” to “B”?
b) [7 pts] What is the wavelength of this photon?
c) [6 pts] What color is the light of this
photon?
E = hf = h(c/![]() )
)
![]() = hc/E
= hc/E
![]() = (6.626 x 10 - 34 Js)(3.0 x 10 8 m/s) / 2.22
eV
= (6.626 x 10 - 34 Js)(3.0 x 10 8 m/s) / 2.22
eV
We need to convert E = 2.22 eV into units of J (or the equivalent) to finish carrying out this calculation
This is yellow light, near the middle of the range of visible light.
(C) 2002, Doug Davis; all rights reserved