
(this page is always under construction)
BackgroundThe FT-MW technique provides high accuracy structural information, allowing determination of bond angles and bond lengths from measurement of the rotational spectra for a particular molecule or weakly bound complex to unparalleled accuracy (eight or more significant figures in bond lengths can be determined in some cases!). The technique of FT-MW spectroscopy, originally developed by T.J. Balle and W.H. Flygare at the University of Illinois Urbana-Champaign in the late 1970's, involves pulsing a gas mixture containing the species to be studied, into an evacuated cavity where it is probed by microwave radiation. The gas mixture, typically a 1-3% mixture of the molecules of interest diluted in 97-99% rare gas (such as Ar or He/Ne) is expanded supersonically through a pulsed valve into the evacuated cavity. This expansion makes it possible to effectively 'freeze out' the weakly bound complexes, providing them with no means by which to fall apart so their rotational spectra can then be measured. The current spectrometer hardware/software design described on these pages is based very closely on the 1991 University of Kiel design. This machine uses software developed at Kiel by Jens-Uwe Grabow which allows the spectrometer to automatically scan large regions of the spectrum unassisted.
Measurement of the rotational spectra of molecules and complexes allows a determination of the parameters known as rotational constants. These rotational constants are inversely proportional to the moments of inertia of the species under study and hence allow a structural determination to be carried out. (The moment of inertia is a property that depends on the mass and the 3-D coordinates of the individual atoms in the molecule, hence you can see the source of the structural information that is available from the measurement of the rotational spectrum). The high resolution afforded by the technique allows hyperfine structure due to nuclear quadrupole splittings, spin-rotation or even spin-spin interactions to be resolved, whereas the high sensitivity often makes observation of isotopes in their natural abundance possible. FT-MW spectrometers are sensitive enough to observe transitions from species with very low abundance in the expansion, for instance rotational transtions due to the 18O13C34S isotopomer of OCS (which has a natural abundance of about 0.00009%) may be measured.
So, in summary, microwave spectroscopy provides us with the structures of molecule to very high precision. We can also obtain information on the environments of certain nuclei, for instance, determination of the electric field gradient at nuclei can provide data on the electronic environments and identify the degree of charge transfer upon complexation. Information on energy barriers to internal rotation of methyl groups or motions of monomers within a weak complex may also be available. This sort of information, both the structural parameters and molecular properties, is of importance in understanding intermolecular forces and how molecules interact with one another. Such interactions have important consequences in understanding interactions in real-life, larger scale systems, such as drug interactions, protein folding, even in nanotechnology.
Description of the instruments:





The picture above shows the inside of the vacuum chamber. At the bottom
left of this picture
is the stepper motor (the thing with the wires coming out of it) that moves the mirror in and out
to tune the cavity to the particular frequency of microwave radiation
being used. The bottom mirror is the only one that moves, the upper one is
fixed. The region between the mirrors is the region into which the
gas pulse expands through the solenoid valve (which would extend into the
chamber by about 8-12 inches through the port on the left of the picture).
Between the mirrors, above and below where the nozzle expansion would be)
are the Stark plates, two steel mesh plates to which
we can apply high voltages of opposite polarity when doing Stark effect
measurements (as described above). The surface of the far mirror can be seen
in this picture - it is a smooth polished aluminium face with a microwave
antenna located at the center (it is reflecting the Stark plate
in this picture and so looks strange!) The box of Kimwipes sitting on the
Stark plate is not an essential element of the spectrometer :-) It is
clearly important to remove any objects from the chamber
before sealing it up and
pumping it down and also to avoid dropping anything down into the diffusion
pumps (which are ideally placed in just the position you need for this
to happen). Incidentally, views like this (i.e. the chamber with the lid off)
are usually bad news since it (a) means there is a problem or (b) I am in
the chamber tinkering which usually results in (a). (The spectrometer
chamber is always kept under vacuum even when the pumps are shut off). As
you can imagine, taking a lid that weighs a few hundred pounds off the vacuum
chamber is not a trivial matter and we have an engine hoist (pictured below)
that we use to perform this task.
(2) Chirped-pulse Fourier-transform microwave spectrometer
Since June 2009, we have a second microwave spectrometer operational, a 480 MHz bandwidth chirped-pulse Fourier-transform microwave spectrometer. It also uses a pulsed supersonic nozzle to introduce sample into the vacuum chamber but, as the name suggests, the bandwidth of this new instrument far exceeds that of the resonant cavity instrument (480 MHz versus less than 1 MHz). The CP-FTMW is shown below.
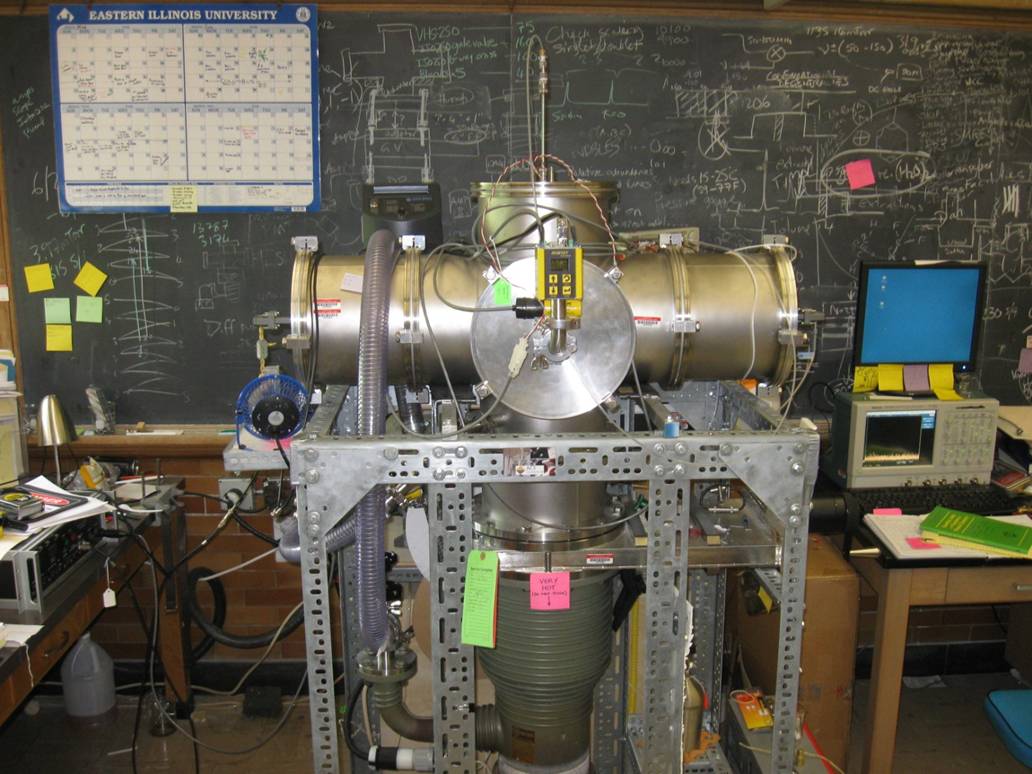
The gas sample is expanded through the same sort of nozzle used on the cavity instrument and can be seen sticking vertically out of the top flange. The gas sample shoots straight down between two microwave horns that are housed inside the vacuum chamber, in the left and right arms of the vacuum chamber. Microwave radiation is broadcast onto the molecules in the gas expansion from the right hand horn, and rotational transitions will then occur in the sample. After a short time (<1 μs) we detect the microwave emission of the molecules with the left horn and then, after some tricks to mix the frequency back down from something in the GHz region to something we can detect (in the MHz region), we send the signal to an oscilloscope (pictured on the desk at the right of the picture) for averaging and Fourier-transformation. The sample then passes into the diffusion pump (the green cylinder at the bottom of the vacuum chamber) and is removed from the chamber ready for the next gas pulse. We can run the nozzle at rates of up to 10 Hz, allowing us to average many thousands of spectra together to allow detection of weak signals.
The clever trick that allows us to increase the bandwidth in this instrument is the use of a chirped-pulse, a significant advance in microwave spectroscopy made by Prof. Brooks Pate at the University of Virginia; by the generation of a very fast (<1 μs) linear sweep of freqencies it is possible for us to generate an envelope of radiation spanning a wide frequency range that can be used to excite rotational transitions in a gas sample, so rather than measuring a spectrum by exciting small (1 MHz) segments at once (as in a resonant cavity spectrometer), the whole rotational spectrum can be collected at once.
Our instrument is a much scaled down version of the UVA instrument allowing us to chirp 480 MHz rather than the full 11,000 MHz that the UVA instrument can manage. The key elements of our spectrometer are shown below: the box at the top of the instrument rack is an arbitrary function generator (AFG) which generates the chirp, in this case a chirp spanning the frequencies from 0 - 240 MHz. (The actual chirp can be seen on the monitor on top of the scope; we actually program the chirp on the scope and then send this function to the AFG). This chirp is then mixed with a single frequency from a microwave synthesizer (the piece of equipment on the bottom shelf of the rack). This gives us a range of frequencies centered around the microwave frequency, so, if we choose a center frequency on the synthesizer of 12000 MHz, we would have a chirp of (12000 ±240) MHz. This means a frequency envelope of 11760 - 12240 MHz will be sent into the vacuum chamber to excite the gas sample. The drawback of this technology is that you are now distributing the microwave power over a wider bandwidth, and the wider the bandwidth, the more amplification that needs to be applied to the polarizing pulse for it to retain its ability to excite the molecules. We use a 10 Watt microwave amplifier to increase this power (this component is the large silver box to the left of the AFG) and this is the single most expensive component of the instrument (about $14,000!)
The spectrometer requires precise timing sequences to be determined (on the sub μs timescale) so that we can properly polarize the gas sample and detect our spectrum. The two boxes under the AFG take care of the timing: the one on the left controls the switch that protects the low noise amplifier on the detection side of the microwave circuit and also controls the 10 Watt microwave amplifier that increases the power of the polarizing MW pulse (the pulse that is used to excite the molecules in the gas expansion). The timing box on the right takes care of synchronizing the AFG (and it controls the other timing box as well); it also sends a pulse to the Iota One (the black box on the shelf below) which controls the gas pulse from the supersonic nozzle.

A close up picture of the lower half of the equipment rack (showing more clearly the MW synthesizer) and the scope is below. The FID, comprising many frequency components, is the green trace on the scope. The light blue trace on the scope is the frequency domain spectrum.

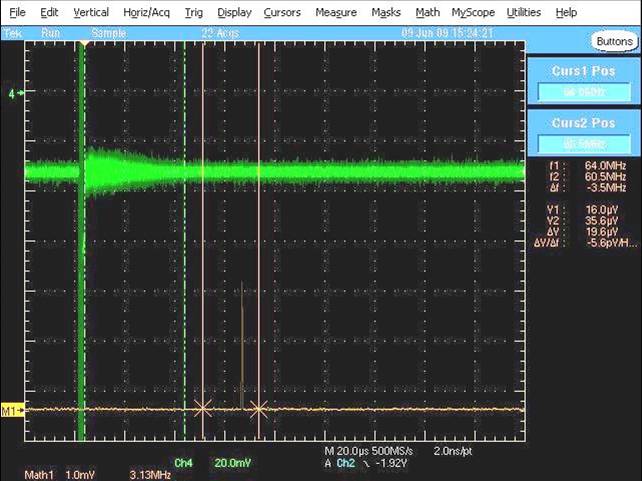
Obviously, intially it took a lot of trial and error to determne the optimum settings of all the components and the best timing sequence, but finally, on June 9th 2009, we saw our first signal. The rotational transition of OCS (carbonyl sulfide) was the first signal we saw (pictured below) and it was a huge relief after spending 9 months putting the spectrometer together (and dealing with vacuum pump issues, vacuum chamber leaks, incompatibilities between "standard" flanges, broken electronic switches and many other adventures). The green trace in the picture below is the time domain signal and the free induction decay (FID, just like in NMR) coming from the OCS microwave emission can be clearly seen, as can the very narrow and intense chirp which is bleeding through to the detection circuit (directly to the left of the FID). The orange/brown trace at the bottom of the screen shows the Fourier-transformed (frequency domain) signal - the small OCS peak can be seen between the two vertical markers; it wasn't much to look at in terms of intensity (it later increased in intensity by about a factor of 20 or more) but at this time we were delighted to see our first signal, even if it was a bit weak.
The earlier projects are systems that I worked on as a postdoc at the University of Michigan. The work carried out at EIU is described below and at the end of this page):
March 2003: We recently finished the assignment of numerous isotopomers of the bromobenzene monomer and this work is due to appear in Volume 257 of the Journal of Molecular Structure. Currently we're assigning isotopic species for 3-butyn-1-ol (including the 18O species in natural abundance, approximately 0.2%) to determine the structure of the conformer that is present in our supersonic expansion. Theoretical calculations to determine the barrier to rotation (and hence the relative stability of other possible conformers) are also underway using the Chemistry department Alpha Tru64 Unix workstation as well as my own 2.8 GHz Pentium 4 PC.
August 2003: We're making our last frantic attempts to get some research done before the Fall classes start again. Currently we're attempting to assign the rotational spectrum of the OCS-CS2 weakly bound dimer.
October 2003: We finally have our Glassman High Voltage high voltage power supplies (EL Series) that will allow us to do Stark effect measurements on some of our more intense OCS/CS2 transitions. This will hopefully give us valuable information on what the quantum numbers are for these transitions and allow us to make some attempts at assigning this spectrum. Using our new cylinder of He/Ne carrier gas (to replace the Ar we have up to now been using) we have managed to improve the intensity of several OCS/CS2 transitions to the point where a Stark effect is possible.
The Ar-bromobenzene weak complex continues to give us headaches - we have made some progress in recent weeks in assigning the hyperfine structure but the quality of the fit indicates something is not right (bad quantum number assignments, wrong transition frequencies or something else weird that we have yet to think of). It seems like we are on the right track with this assignment but we're still awaiting the breakthrough :-)
December 2003: Preliminary searches for the DME-OCS complex have been carried out in recent weeks leading to several promising transitions having been located. Mixing tests are underway (to test that these lines require both DME and OCS!) and the high voltage connectors (that will enable us (eventually) to carry out Stark effects on these transitions) have been ordered (Reynolds Industries Series 521 plugs, P/N 167-3516). Once we have the power supplies hooked up and can do some Stark effects we're hoping this will give us some hints as to the quantum numbers of the transitions we have found and will aid in the spectral assignment.
During the Christmas break, we measured the rotational spectra of the normal isotopic species and six isotopically substituted species of cyclopropyl carbinol (a cyclopropane with a CH2OH group on one of the ring carbons). This allowed a heavy atom structure determination, and that, coupled with the calculations that Josh carried out on this molecule will eventually be put into a manuscript. Additional work on dipole moment measurement and searches for higher energy conformers is underway. In addition to the experimental work, Josh is keeping both the lab PC and the departmental workstation busy on DME-OCS, DME-HCCH and DME-HCN calculations.
February 2004 : The power supplies are finally working and have already assisted in the assignment of a weakly bound complex spectrum! Thanks to Jim Wentz of the Computer Electronics Electrical Services at the School of Chemical Sciences at the University of Illinois, Urbana-Champaign who fitted the connectors and helped us with getting "standard" connectors to fit in "standard" feedthroughs (which initially did not fit). We got the OCS electric field calibration carried out with our new power supplies (on Jan. 29th) and then did a Stark effect on two of the most intense lines from the DME/OCS search. As soon as those were done and plotted, we had the information that we needed to assign that dimer spectrum and the normal isotopic species spectrum is now measured :-) We're now working on getting the dipole moment measured properly and then we're on to thinking about isotopes.
April 2004 : The measurement of the DME-OCS isotopomer spectra is finished and we have completed the determination of the structure of this complex. High on our list of things to do is the preparation of our talks for the Columbus spectroscopy meeting in June 2004. We have also started upon the upgrade of the spectrometer to a newer (and hopefully more efficient) design. This involves a major upgrade of the hardware and software used to drive the spectrometer. This project will continue for the summer (and beyond) although the current configuration of the spectrometer will be used to continue our experimental studies until the time comes for us to switch over and test the new design. Currently we are experiencing serious problems with our Dell Precision 650 workstation, which is freezing up right at the start of the boot up process and giving us error messages about possible graphics card failure. A replacement ATI Fire GL X1 128 MB card from Dell would not work at all; even a replacement of the entire motherboard and a new ATI card gives us similar problems. It is looking likely that there is a serious conflict with the graphics card somewhere. Current hardware count: 3 motherboards, 2 CPU's, 2 sets of memory, 2 power supplies, 4 video cards (the last one an NVIDIA QuadroFX500).
June 2004 : We're working on more DME complexes - DME-CO2 has been assigned and is currently being written up and at present we're working on assigning the DME-CS2 complex. This system is particularly interesting (and difficult) since we seem to have a tunneling motion that interconverts between two equivalent structures of the complex. Spectroscopically this complicates matters significantly because some of the transitions are perturbed considerably with the lines being split into a doublet and those components being displaced to high and low frequency. We also managed to get some Stark effects carried out (finally!) on the OCS-CS2 transitions that we had (see the entry above for August and October 2003) and we assigned this spectrum. We've now measured the normal and four additional isotopic spectra in natural abundance and have added this one to the list to write up. Of course, the main highlight this month was the week we spent at the 59th Ohio State University International Symposium on Molecular Spectroscopy. The official picture archive for the conference is online here. A few pictures of the EIU representation at the meeting are here.
August 2004 : Currently we're working on the DME-HCCH complex (the DME-CS2 is on hold while we try to figure out how to obtain the barrier to the internal motion from the inversion splittings and the OCS-CS2 is being written up). We have found several transitions for the DME-HCCH complex and have a tentative assignment (we have a-type K = 0 and K = 1 lines for the 2-1, 3-2, 4-3 and 5-4 transitions but no K = 2 lines and no c-type transitions yet. We're expecting some internal motion problems just as we saw in the DME-CS2 complex although haven't yet identified the tunneling doublets (if they exist :-) Prof. Walther Caminati at the University of Bologna in Italy very kindly supplied us with the Fortran code for a flexible model program which he has used in the past to fit the barrier to inversion for similar tunneling motions in DME complexes with rare gas atoms. This gave us the excuse we've been looking for to justify the purchase of the Absoft ProFortran Compiler Suite v.8.2. This will allow to compile and run this program and determine the barrier to the inversion motion for DME-CS2 (once we have figured out a tunneling pathway and potential energy function for the motion!)
Newby finally left this month to go to graduate school at Purdue University (and the new Wal Mart in West Lafayette), leaving behind several hundred megabytes of output files from his numerous Gaussian jobs.
Michal Serafin joined the group at the start of the Fall 2004 semester. Michal has begun work on modeling the dimethyl sulfide-CO2 complex using Gaussian 98.
September 2004: Michal continues to work on calculating the possible structures for the DMS-CO2 complex and has also been spending time predicting rotational spectra.
October 2004: Michal is preparing his poster on DMS-CO2 for the upcoming Third Annual Undergraduate Research Celebration (to be held in the Chemistry Department, 3rd floor, 5.30pm on November 1st). We're also in the process of systematically applying the ORIENT semi-empirical model to several complexes of DME to see how well it is capable of predicting their structures. The DME-CO2 paper was accepted this month for publication in J. Phys. Chem. and should appear in a January 2005 issue of this journal.
November 2004: We're in the process of measuring the 13C isotopomers for the DME-HCCH species (in natural abundance).
January 2005: We spent some time over the Christmas break searching for the DMS-CO2 spectrum although this was made difficult by some hardware problems that affected the autoscan facility. Mike will be returning to the lab this semester and one of his first jobs will be to do the basis set superposition error (BSSE) calculations on this complex. The OCS-CS2 work that we submitted to the Royal Society of Chemistry journal Phys. Chem. Chem. Phys. in September was accepted for publication in December and was published as an Advance Article on the web January 10th 2005 (article number b414897e).
April 2005: We're still proceeding slowly with the spectrometer upgrade :-) Mike is continuing his calculations on the DMS systems and is involved in the modeling of ionic complexes. The DME-HCCH manuscript was published in J. Phys. Chem. A. 109, (2005), 5316.
May 2005: Summer at last! It looks as though we have an assignment for the DME-HCF3 weakly bound complex (dimethyl ether complexed with fluoroform (trifluoromethane)). We've found a number of a-type transitions but they are all split to varying degrees (possibly by internal motions of the DME methyl groups in addition to the internal rotation of the trifluoromethane subunit) e.g. the K = 0 lines are apparently split into quartets while the K = 1 lines all seem to be doublets. In talking to Prof. Caminati at Columbus in June 2005, we found that he has assigned this complex and (fortunately) has already figured out the internal motion splittings in the spectrum :-)
June/July 2005: In searching for the DMS-OCS spectrum, we have an assignment for the DMS-Ne complex (we've so far got the normal isotopic species and the 22Ne species). Also this month was the 60th International Symposium on Molecular Spectroscopy at the Ohio State University, meaning we had far too many doughnuts and coffee during the week of June 20-24.
We have also recently assigned a couple of isotopic species for the DME-CS2 complex (in the hope that we can sort out unambiguously the structure and tunneling pathway) and have a tentative assignment for HCCH-HCF3 (the acetylene-fluoroform (trifluoromethane) complex); there are lots of lines observed here so clearly some more internal motion to sort out.
August-October 2005: Michal measured the DCCD-HCF3 spectrum. This is also complicated by significant amounts of internal motion splitting but the ease with which we were able to locate the spectrum for the deuterated species tells us that our predicted structure is quantitatively close to the true structure. Mike also started searching for the less symmetric OCS-HCF3 complex, which we hope will give us some insight into the nature of the splittings in the HCCH-HCF3 spectrum.
November 2005: The spectrum for the normal isotopomer of the OCS-HCF3 species is assigned and considerably less complex than the spectrum for the HCCH-HCF3 species.
January 2006: We're working on dipole moment measurements of the OCS-HCF3 species and also on the measurement of conformers of some silane samples supplied to us by Prof. Gamil Guirgis of the College of Charleston, South Carolina.
May 2006: We're continuing measurements on the silanes sent to us by Prof. Guirgis. Once that's done Mike will be working on measuring isotopic spectra for the OCS-HCF3 complex (assuming the O13CS isotopically enriched sample that went missing somewhere between Icon Isotopes and EIU in late March is located).
June 2006: One of our projects early this month involved us trying (three times) to synthesize some deuterated fluoroform (DCF3). Unfortunately we failed to get significant (probably any) deuteration and ended up buying some commercial isotopically enriched DCF3. Here is a rare picture of physical chemists in the lab doing real chemistry (Mike's stirring the hexanes/liquid nitrogen slush bath that we used to condense and keep the fluoroform a liquid during the course of the reaction - trying to do chemistry with substances that boil below -80°C is not a trivial matter!). The other main event in early June was that the O13CS isotope finally arrived (some two months after it was shipped). Apparently its journey from New Jersey to EIU involved stops in Indianapolis and Decatur before it was finally located in North Carolina (although quite what it was doing there we never really quite got to the bottom of). Mike managed to get this spectrum measured in time for his talk at the Columbus meeting - his first talk at a meeting! Here's a picture of us at the Columbus picnic - from left to right: Newby, Dr. S. Peebles, Larry Keniley, Mike Serafin. Here's another with Dr. S. Peebles replaced by Dr. R. Peebles.
July 2006: Mike's gone for a week's vacation and before we knew what was happening Rebecca had laid claim to the spectrometer for a week to search for some complexes of CHCl2F (dichlorofluoromethane). Pictures of the current state of construction of her own cavity ring-down spectrometer can be found here.
In late July (a few hours after Mike finished for the Summer) we assigned the CO2-HCF3 complex; this spectrum is complicated by the existence of A and E states (arising from internal rotation of the HCF3 subunit), but also exhibits b-type transitions. These transitions should be forbidden due to symmetry but occur because of a mixing of the K-1 asymmetry doublets (which is permitted by the internal rotation motion). There is evidence for this sort of behavior in the literature and we are working to tighten up the quality of our fit of the observed transitions to within our measurement accuracy.
September 2006: We're currently measuring the rotational spectra of the gauche conformer of the H2Ge(CH3)(c-C3H5) cyclopropyl methyl germane (CMG) compound supplied to us by Prof. Guirgis. This has five Ge isotopes (70Ge, 72Ge, 73Ge, 74Ge and 76Ge). We apparently have A and E states for all the observed transitions (arising from the internal motion of the methyl group) as well as nuclear quadrupole hyperfine structure in the quadrupolar 73Ge isotopomer. Unfortunately we had very little sample and exhausted it before we could complete all the measurements.
November 2006: Just before the Thanksgiving break we got an a-type assignment on the methyl fluoride-OCS complex. This means we have a series of three fluorinated methane complexes with OCS (CHF3, CH2F2 and CH3F). All we need now is to find some b-type transitions.
March 2007: Mike's working on trying to find more methyl fluoride-OCS transitions while Jon's been working on doing Morokuma decomposition calculations using GAMESS and NBO analyses using Gaussian. There should also be some more measurements to do on H2Ge(CH3)(c-C3H5) since we are expecting to receive another sample soon so Jon's predicting the quadrupole coupling constants for the 73Ge isotope to aid in deconvolution of the internal rotation and nuclear quadrupole hyperfine components.
April 2007: Mike found the b-type lines for the MeF-OCS complex finally! Now we have to see if we can find any additional (E-state) lines that can be attributed to the internal rotation of the MeF subunit about its symmetry axis. With the arrival of more of the CMG sample from Dr. Guirgis we were able to finish measurements of the nuclear quadrupole hyperfine structure for the 73Ge species. The quadrupole coupling constants calculated by Jon Murray for this species proved to be in perfect agreement with the observed values and hence made assignment of this quadrupole structure quite straightforward.
May-August 2007: We're visiting Dr. Steve Cooke's lab at the University of North Texas for the summer where we're hoping to look at some Pt and Pd containing species, with a break in mid-June to go to the 62nd International Symposium on Molecular Spectroscopy at Ohio State University (where Mike will be presenting a paper titled "Characterization of Weakly Bound HCF3-OCS, H2CF2-OCS, H3CF-OCS, and HCF3-CO2 Van Der Waals Complexes by Ab Initio Calculations and Microwave Spectroscopy"). Some pictures of the EIU gang at the Columbus picnic: Dr. S. Peebles with Dr. Steve Cooke, Dr. R. Peebles, Amanda Steber, Mike Serafin, Mike listens intently to the introductions, Newby joins the EIU table at the picnic, Amanda enjoying her ice-cream.
November 2007: It's been a busy semester so far, we managed to get the 13C-DFM-OCS spectrum assigned in natural abundance (finally, after several attempts) and were able to get a decent structure on this complex. We're also in the process of putting together a pulsed-discharge nozzle.
May 2008: Mike defended his Masters thesis.
June 2008: It's time for the Columbus spectroscopy meeting again. Pictures are here.
August 2008: We're currently testing a new pulsed discharge nozzle (PDN) which we hope to use to generate some new radical and cationic species. And since our NSF-RUI proposal was also funded last month, we're also working on the details of a new instrument with which to pursue additional studies of ions and ionic complexes.
October 2008: I'm on sabbatical for this academic year and I am in the process of building a new instrument. We have been very busy this semester getting things ordered as well as continuing our research efforts and also trying to make progress on writing up some of the collaborative projects that we have been working on in the last couple years.
March 2009: Both Ashley and Amanda won awards for their research and presented at Sciencefest (and Amanda also presented her poster at the EIU Showcase event) - Ashley got a Graduate Student Investigator (GSI) Award and Amanda got a Scholars in Undergraduate Research at Eastern (SURE) Award (in fact being ranked top of the SURE Award winners by the committee). Ashley was also awarded a Williams Travel Award by the Graduate School to fund her trip to present her work at the Columbus meeting in June 2009.
April 2009: Amanda and several other undergraduate students from EIU presented their work at the University of Illinois Women Chemists Committee Undergraduate Research Symposium. Pictures from this event are here.
May 2009: The new instrument is coming along and we're in the final stages of working out the timing and hope to see a signal early in the summer. It's going to be busy in the lab with Amanda Steber, Ashley Osthoff, Dan Obenchain and John Sexton all working on various aspects of the NSF funded work. The group (as of the middle of Spring 2009) is pictured below: (left to right: Dr. Rebecca Peebles, Ashley Osthoff, Amanda Steber, Dan Obenchain, Dr. Sean Peebles. Behind everyone is our new chirped pulse FTMW spectrometer, which we hope will be able to measure between 200 and 400 MHz of rotational spectrum at once, in contrast to our other resonant cavity instrument, which can measure about 1 MHz at a time).

June 2009: June's always busy and we started off with a trip (with Amanda and Dan) to Prof. Brooks Pate's lab at the University of Virginia to work out the final issues with our new chirp. We also got a couple broadband spectra measured while there to keep us busy in the assignment department :-)
Big news this month: We saw the first OCS signal on the new instrument! And on June 19th, the day before setting off for the Columbus meeting, we assigned our first spectrum on this instrument. Dan assigned the spectrum for the trans-gauche conformer of the 3,3-difluoropentane molecule. Huge thanks to Brooks Pate and to his students at UVA for being willing to put up with our visits - thanks to Matt Muckle, Danny Zaleski and particularly Justin Neill for all their generous help with getting our new instrument functional.
Everyone's been busy this month - Amanda has been assigning diethylgermane spectra and worked out how to use JB95 which was also used to assign the CHClF2-HCCH spectrum. We'll once again attempt to assign the elusive CHClF2-CO2 spectrum once we return from Ohio State. John and Ashley have been busy working on that assignment (which is difficult since the entire spectral region is full of lines due to the rich spectrum from the CHClF2 monomer, its isotopologues and vibrationally excited states) as well as making significant progress in getting the IR laser set up and aligned. Dan's working on getting the hang of the new chirp instrument by assigning some test molecules and getting the timing and experimental sequences figured out.
The Columbus meeting was huge fun as always - Ashley, Amanda and myself all gave talks this year, and this was a first for both Amanda and Ashley. The group (as of June 2009) is pictured below (taken at The Cheescake Factory, Columbus, OH) - from left to right: Amanda Steber, Dr. Sean Peebles, Dr. Rebecca Peebles, Ashley Osthoff, John Sexton, Dan Obenchain.
July 2009: Amanda, Rebecca and myself visited the Far IR beamline at the Canadian Light Source synchrotron facility in Saskatoon, Canada for a week, to make additional measurements. This coincided nicely with the annual Taste of Saskatchewan festival :-)
August 2009: Prof. Jung-Jin Oh (from Sookmyung Women's University in Seoul, South Korea) visited our lab for several days just before classes started again to collect data using our two microwave spectrometers. We measured and assigned spectra for 1-bromobutane, 2-bromobutane and also for several conformers of n-butylsilane while he was here.
October 2009: The Henry Dreyfus Teacher-Scholar Award proposal that I submitted back in the summer was funded so we should get some money to buy an ablation laser at some point soon! Here's the EIU press release.
Also in late October Dan presented a poster detailing our new instrument and the 3,3-difluoropentane results at the 44th ACS Midwest Regional Meeting in Iowa City.
November 2009: Dan presented his research at the annual Chemistry Department Student Research Celebration. Some pictures of this event are here. Over the last couple of months we have also been working on refining the operation of our new CP-FTMW spectrometer (using difluorosilylisocyanate (SiHF2NCO, synthesized by Dr. Gamil Guirgis) and also developing our ion sources.
December 2009: Ashley successfully defended her thesis and is hoping to finish up all the corrections and paperwork before the end of the semester. At the same time as Ashley was in her defense, Dan gave an enthusiastic 10 minute presentation on his summer research as part of the College of Sciences Summer Undergraduate Research Colloquium.
March 2010: We finally ordered our Nd:YAG laser (funded by the Dreyfus Foundation grant) so hopefully that will be here some time in early April. Also this month Dan won a SURE (Scholars in Undergraduate Research at Eastern) Award, meaning that he will be spending a good bit of time in the next few weeks presenting posters at various EIU events (ScienceFest, EIU Foundation Legacy Student Showcase and Showcase EIU). Unfortunately, also this month, our Rb frequency standard stopped working and had to be sent back to the company to have the Rb crystal replaced; this meant there were about 2 months where we were unable to run anything on the new chirped-pulse instrument.
April 2010: Dan gave his poster at the EIU Showcase event. On the same day as that presentation, our new Nd:YAG laser arrived in the lab. Dan presented his research in an oral presentation at the Undergraduate Research Symposium at the University of Illinois, Urbana-Champaign this month, and was selected as the Best Oral Presenter by the judges.
May 2010: Rebecca and I started the summer with another visit to the Canadian LightSource facility in Saskatoon, Canada. We had a productive 5 days of beam time although there were some creative problems with the storage ring. In late May the chirp instrument was finally back up and running with the return of our Rb frequency standard, and we saw a weakly bound complex transition (belonging to the CHClF2-H2O complex) on it for the first time.
June 2010: Dan gave his first presentation at the 65th International Symposium on Molecular Spectroscopy at the Ohio State University in Columbus, OH. Amelia and Brandon also attended the meeting, with Lena unfortunately having to remain at EIU since she was enrolled in a summer Physics course. Official conference pictures from the 2009 and 2010 meeting are here.
September 2010: We saw our first radical signal on September 22nd! We measured a set of transitions belonging to the OH radical (produced in an electric discharge of water vapor entrained in an Ar carrier gas). These transitions were seen on our resonant cavity instrument and now we want to try to improve the intensity such that we can see it on the chirped-pulse instrument.
December 2010: Latest component to fail - the low noise amplifier :( We sent this back to Miteq for evaluation. One of the early stages of the amplifier was damaged and it was replaced (still under warranty fortunately) but this cost us about another 2 months with no instrument.
April 2011: Lena and Amelia attended the Undergraduate Research Symposium at the University of Illinois. Amelia won the prize for the Best Poster Presentation.
June 2011: Dan and Rebecca gave talks at the 66th International Symposium on Molecular Spectroscopy at the Ohio State University in Columbus, OH. Amelia and Lena also attended but did not present this year. This year it was Cori and not Lena that had to stay behind at EIU to take summer classes. Shortly after getting back Dan finally came up with a scheme to coax multiple chirps per gas pulse from the equipment that we have. It works well although there are clearly still a few issues to be ironed out since we are still seeing a little loss of signal intensity on going from single to multiple chirps. Nonetheless, a big step forward :)
July 2011: After a lot of failed experiments stretching back over a year, we finally saw the first radical complex on the chirp instrument. Dan managed to get a quite respectable signal for the OH radical complexed with water (previously studied by the Endo group and the Leopold group). This gives us great faith in our instrument's ability to see weak complexes involving exotic species such as radicals and was a massive step forward in that instrument's development.
August 2011: Right before classes started, the departmental Linux workstation (which we rely upon to run Gaussian 03) decided to fail. The motherboard had died and with this machine having been declared obsolete a few months after we bought it in 2007 things looked bleak. We did manage to track down a replacement board on eBay for under $100 so things are working again but we're not sure how long for... A replacement machine is in the pipeline. The lab also welcomed a new member this month, courtesy of Lena and Cori (he's not particularly research-active it has to be said :)
September 2011: Jihyun Kim (from Prof. Jung-Jin Oh's group at Sookmyung Women's University in South Korea) visited the lab for 3 weeks and made measurements on several compounds, both on the resonant cavity instrument and on the chirped-pulse instrument.
April 2012: Lena won the prize for the Best Oral Presentation at the Undergraduate Research Symposium held at the University of Illinois Urbana-Champaign with her talk on the trifluoromethane...vinyl fluoride complex.
June 2012: The group made the usual trip to the Columbus meeting. Cori, Lena, Yasser, and Anthony all attended and Lena gave a talk on her work on the vinyl fluoride...trifluoromethane complex.
August 2012: The National Science Foundation Research at Undergraduate Institutions grant was officially renewed this month! Nathan also joined the group.
November 2012: Nathan was awarded an Undergraduate Research, Scholarship and Creative Activities (URSCA) award to fund working on installation and testing of the electron gun in the Spring 2013 semester.
January 2013: Anu and Ashley joined the group.
March 2013: Nathan was awarded a SURE (Students in Undergraduate Reseach at Eastern) Award.
April 2013: Nathan attended (and presented a poster) at both the American Chemical Society (ACS) National Meeting in New Orleans and the National Conference on Undergraduate Research (NCUR) meeting in La Crosse, Wisconsin. These meetings were back to back and he presented different projects at both meetings.
May 2013: Cori, Lena, Ashley and Nathan all graduated! Lena is finally leaving the lab - she's off to the University of Illinois Chicago Medical School. We'll definitely miss her, and especially her mom's food :-) Cori and Nathan will be around for the summer, before Cori heads back to Florida to Disney World for a year, and Nathan begins his PhD at the University of Michigan, Ann Arbor. Ashley has to stay behind, since she is now enrolled in the Masters program at EIU. Picture below shows Cori, Lena, Anu, Ashley and Nate scaring the traffic on Lincoln Avenue the day before graduation.

September 2013: Our work on the weakly bound dimer between fluorobenzene and acetlyene was published in Physical Chemistry Chemical Physics. The reasonably large induced dipole moment that we observed in this complex led us to ponder the possibility of seeing a rotational spectrum for the benzene...acetylene complex (which of course is formed from two non-polar monomers and hence can be expected to have a dipole moment that arises purely from induction effects). The fluorobenzene...acetylene paper is here.
We also finally got an up to date group photo. It's been hard to get everyone in the same place at the same time. Back: Anu, Ashley, Dr. Sean Peebles, Dr. Rebecca Peebles. Front: Nathan, Lena, Cori. Thanks to Ruth Riegel for making the most of this scary bunch and getting a nice picture.

March 2014: Our work on the weakly bound dimer formed between benzene and acetlyene was published in Physical Chemistry Chemical Physics. This work, in collaboration with the group of Professor Brooks Pate at the University of Virginia (who supplied us with the scan of the rotational spectrum for the complex between these two non-polar monomers), provided the first high-resolution spectroscopic study of this prototypical CH...π bonded system. The paper can be found on the PCCP website here and was designated a "Hot PCCP Article" by the journal.
May 2014: Ashley successfully defended her Masters thesis. Anu is writing and hopes to finish in July or early August. Undergraduates Sarah Stettner and Bailey Luce joined the lab for the summer, supported by the National Science Foundation Research at Undergraduate Institutions grant.
Summer 2014 group photo (OK, so it's slightly fuzzy...), from left to right: Anuradha Akemeemana, Sarah Stettner, Bailey Luce, Dr. Sean Peebles, Lester, Rachel Dorris, Ashley Anderton, Dr. Rebecca Peebles.
June 2014: Ashley, Rachel, and Drs. Sean and Rebecca Peebles attended the 69th International Symposium on Molecular Spectroscopy. This was the first time that the meeting has been held outside the Ohio State University in Columbus, OH, having moved to the University of Illinois at Urbana Champaign after an incredible 68 consecutive years in Columbus. We're pleased to report that the supply of coffee and donuts throughout the week of the meeting, a defining feature of this symposium for many years, was uninterrupted and plentiful, allowing for a very enjoyable and productive meeting. The Wednesday evening picnic also supplied abundant food, drink and an excellent opportunity to catch up with sepectroscopic colleagues from all over the USA and the world, including several EIU graduates (Josh Newby, who will be starting as an Assistant Professor in the Chemistry Department of Hobart and William Smith Colleges in Fall 2014, Dan Obenchain (currently doing a PhD with Stew Novick at Wesleyan University in Connecticut), and Amanda Steber (in the final stages of her PhD at the University of Virginia). Dan came back to EIU and spent a valuable 5 days in the lab after the meeting (although the timing of the World Cup likely reduced our effectiveness somewhat for certain parts of the day).
August 2014: Anu successfully defended her thesis. As of January 2016, Anu is enrolled in a Forensic Chemistry Ph.D. program at the University of Central Florida.
January 2015: Justin Kang, from Oberlin College in Ohio, spent a few weeks in our lab for his winter term project. During that time we made significant progress on the asssignment of the 1,2-difluorobenzene...HCCH spectrum.
June 17th 2015: In the already frantic run-up to the spectroscopy conference, our preparations were complicated by the arrival of a stray kitten that had apparently hitched a ride home from campus underneath the car. "spcat" is now an official member of the group whose main assignments include sleeping, eating, making weird chirping noises and providing stress relief.
June 22-26th 2015: We all attended the 70th International Symposium on Molecular Spectroscopy, which is now hosted at the University of Illinois Urbana-Champaign. Rachel Dorris presented her work on the vinyl fluoride...difluoroethylene complex. After the meeting we all attended the Far IR Workshop, organized by the Canadian Light Source. After returning from the meeting, Heesu Jang and Dr. Soohyun Ka (from the research group of Prof. Jung-Jin Oh of Sookmyung Women's University in Seoul, South Korea) visited our lab for a couple weeks to make measurements.
February 26th 2016: Our new 6 GHz Tektronix oscilloscope finally arrived! After much back and forth with university administration to get approval to spend the money we were finally able to get the order placed and the scope arrived today. We hope that with implementation of the long planned 2-8 GHz frequency extension to our chirped-pulse instrument, this new scope will allow direct detection of transitions up to 6 GHz, simplifying our data collection circuitry and streamlining our ability to identify new molecular signals.
Vacancies: We're always looking for undergraduate research students so if you want to
come by and see what we do then feel free to drop by my office (Room 3420
in the Physical Science Building) or my lab (Room 2443), or you can email me
at sapeebles@eiu.edu or call me at (217) 581-2679. Below is a list of undergraduate researchers
and an idea of the projects that they have worked on.
National Science Foundation funded Summer research: There is funding available to support undergraduate research students in Summer 2016. Please contact either myself (sapeebles@eiu.edu) or Dr. Rebecca Peebles (rpeebles@eiu.edu) if you are interested in learning more.
 Elizabeth D. Slagle (Summer 2003): carried out ab initio calculations on conformers of 3-butyn-1-ol, hydrocinnamaldehyde and o-anisaldehyde. These molecules all have the potential to form intramolecular hydrogen bonds. This study allows the order of stability to be deduced for the various conformers and provides predictions of the spectroscopic parameters of interest needed to initiate a search for the rotational spectra of these species. Her work on 3-butyn-1-ol was included in a manuscript on the rotational spectrum and ab initio calculations on this molecule (this paper appeared in the Journal of Molecular Structure in May 2004). Elizabeth graduated from EIU in 2003 and is apparently now living in Alabama with her husband, Kelly (after brief spells living in Florida and Texas). [Publications]
Elizabeth D. Slagle (Summer 2003): carried out ab initio calculations on conformers of 3-butyn-1-ol, hydrocinnamaldehyde and o-anisaldehyde. These molecules all have the potential to form intramolecular hydrogen bonds. This study allows the order of stability to be deduced for the various conformers and provides predictions of the spectroscopic parameters of interest needed to initiate a search for the rotational spectra of these species. Her work on 3-butyn-1-ol was included in a manuscript on the rotational spectrum and ab initio calculations on this molecule (this paper appeared in the Journal of Molecular Structure in May 2004). Elizabeth graduated from EIU in 2003 and is apparently now living in Alabama with her husband, Kelly (after brief spells living in Florida and Texas). [Publications]
Josh J. Newby (Fall 2003 - Summer 2004): Josh was involved in some of the early work that got us moving into our first experimental studies of dimethyl ether (DME) complexes. He did ab initio and experimental work on DME-OCS, DME-CO2, DME-CS2, DME-HCCH, OCS-CS2, cyclopropyl carbinol, DMS-OCS. After leaving EIU, Newby joined the group of Dr. Tim Zwier at Purdue University doing laser spectroscopy, getting his Ph.D. in 2009. Newby gave his first talks (WI06 and FD02) at the International Symposium on Molecular Spectroscopy at Ohio State University in Columbus, OH in June 2007. After his Ph.D. he worked in the lab of Prof. Mary Wirth at Purdue, getting some Analytical Chemistry experience before spending some time as a Visiting Professor at Swarthmore College. In Fall 2014 he will take up a tenure track position at Hobart and William Smith Colleges. [Publications]
Michal M. Serafin (Fall 2004 - Summer 2008): Mike started with some ab initio calculations on DMS-HCCH and DMS-CO2, and moved on to carry out experimental measurements on DME-DCCD and DCCD-HCF3. In Fall 2005 he searched for and assigned the spectrum of his own complex (the OCS-HCF3 dimer). Additional measurements on several isotopomers of OCS-HCF3 and the assignment of the spectra for some of the silane samples provided by Prof. Gamil Guirgis were carried out in Spring 2006. In the Summer of 2006 he extended his experimental studies to difluoromethane (DFM) complexes, assigning DFM-OCS before coming back to CO2-HCF3 (which turned out to be quite interesting - more details are given under the "July 2006" entry in the previous section). Mike also came with me to the University of North Texas during a very productive few weeks in Summer 2007 to work in Dr. Steve Cooke's lab. After successfully defending his Masters thesis in Spring of 2008, he moved his studies to
the Medical Academy of Gdansk, Poland. [Publications]
 Jon M. Murray (Fall 2006, Spring 2007) - worked on measurements on the cyclomethylgermane compound obtained from Prof. Guirgis; also carried out calculations of the electric field gradient at the Ge nucleus to provide predictions of the nuclear quadrupole hyperfine structure in the 73Ge spectrum; Natural Bond Order analysis and Morokuma decomposition analysis calculations on the fluorinated methane series of complexes. Jon's currently attending Midwestern University in Downer's Grove, Illinois. [Publications]
Jon M. Murray (Fall 2006, Spring 2007) - worked on measurements on the cyclomethylgermane compound obtained from Prof. Guirgis; also carried out calculations of the electric field gradient at the Ge nucleus to provide predictions of the nuclear quadrupole hyperfine structure in the 73Ge spectrum; Natural Bond Order analysis and Morokuma decomposition analysis calculations on the fluorinated methane series of complexes. Jon's currently attending Midwestern University in Downer's Grove, Illinois. [Publications]
Amanda L. Steber (Fall 2008, Spring 2009, Summer 2009) - Amanda has worked in Dr. Rebecca Peebles' group since Fall 2005 although since Fall 2008 has been working on analyzing some of the broadband microwave spectra of diethylsilane that we collected at the University of Virginia. Isotopic substitutions of all three conformers of this molecule have enabled a full heavy atom structure. Amanda presented this work at Columbus in Summer 2009 and, after giving us one last summer working in our research lab, went off to the University of Virginia to pursue a PhD in the group of Prof. Brooks Pate. One of Amanda's many useful achievements in the lab was to finally figure out and teach me how to use the JB95 assignment program. Knowing I'd instantly forget how to use it as soon as she left EIU, she was insightful enough to provide us with her definitive written guide to using the program :-) Amanda gave her first presentation as a member of the Pate group at the International Symposium on Molecular Spectroscopy in June 2010. In November of 2014, Amanda successfully defended her Ph.D. at the University of Virginia. Amanda was awarded a Louise Johnson fellowship as a postdoc in the group of Prof. Melanie Schnell at the Max Planck Institute for the Structure and Dynamics of Matter (specifically The Hamburg Center for Ultrafast Imaging), Hamburg. [Publications]
 Daniel A. Obenchain (Spring 2009 - Summer 2011) - Dan worked initially with Amanda analyzing some of the diethylsilane broadband spectra but was also heavily involved in the construction of the new CP-FTMW instrument. As well as being instrumental in getting the new chirped-pulse spectrometer working (by spending many hours working to optimize the timing) and assigning the first spectrum (3,3-difluoropentane) from the new instrument, Dan dealt with many more mundane jobs such as building instrument support frameworks and an important early achievement was the construction of a heavily soundproofed box to quiet our irritatingly noisy vacuum pump and keep everybody sane in the lab. For the last year or so he has been working hard on developing reliable ion sources; we've so far seen some exotic species generated by pulsed discharge nozzle (such as benzyne and fluorodiacetylene - produced from precursors such as benzene and its derivatives - and also in Fall 2010, OH radical). In addition he has assigned spectra for various conformers of divinyl silane, difluorodivinyl silane and 1-fluorosilacyclopentane from samples sent by Gamil Guirgis and also played a significant role in the assignments of broadband spectra sent to us from UVA (such as CHBrF2-HCCH and CHBrF2-H2O). Dan graduated from EIU at the end of Spring 2011, and after one more summer of frantic research at EIU (seeing our first radical complex on the new baby chirp instrument in the process), moved on for his PhD in the group of Professor Stewart Novick at Wesleyan University in Connecticut. In March 2016, Dan received a prestigious Alexander von Humboldt research fellowship that will support him working in the lab of Prof. Jens-Uwe Grabow at the University of Hannover (story on News@Wesleyan page). [Publications]
Daniel A. Obenchain (Spring 2009 - Summer 2011) - Dan worked initially with Amanda analyzing some of the diethylsilane broadband spectra but was also heavily involved in the construction of the new CP-FTMW instrument. As well as being instrumental in getting the new chirped-pulse spectrometer working (by spending many hours working to optimize the timing) and assigning the first spectrum (3,3-difluoropentane) from the new instrument, Dan dealt with many more mundane jobs such as building instrument support frameworks and an important early achievement was the construction of a heavily soundproofed box to quiet our irritatingly noisy vacuum pump and keep everybody sane in the lab. For the last year or so he has been working hard on developing reliable ion sources; we've so far seen some exotic species generated by pulsed discharge nozzle (such as benzyne and fluorodiacetylene - produced from precursors such as benzene and its derivatives - and also in Fall 2010, OH radical). In addition he has assigned spectra for various conformers of divinyl silane, difluorodivinyl silane and 1-fluorosilacyclopentane from samples sent by Gamil Guirgis and also played a significant role in the assignments of broadband spectra sent to us from UVA (such as CHBrF2-HCCH and CHBrF2-H2O). Dan graduated from EIU at the end of Spring 2011, and after one more summer of frantic research at EIU (seeing our first radical complex on the new baby chirp instrument in the process), moved on for his PhD in the group of Professor Stewart Novick at Wesleyan University in Connecticut. In March 2016, Dan received a prestigious Alexander von Humboldt research fellowship that will support him working in the lab of Prof. Jens-Uwe Grabow at the University of Hannover (story on News@Wesleyan page). [Publications]
 John M. Sexton (Summer 2009) - John worked in the lab for 10 weeks in Summer 2009, having worked for Dr. Rebecca Peebles for 3 weeks as part of the Research Rotation course during the Spring 2009 semester. John worked on a variety of projects in both labs, including working to align the IR laser (part of Dr. R. Peebles' rapid-scan mid-IR spectrometer) and also assigning broadband scans (collected at the University of Virginia) for the CHClF2-HCCH complex. [Publications]
John M. Sexton (Summer 2009) - John worked in the lab for 10 weeks in Summer 2009, having worked for Dr. Rebecca Peebles for 3 weeks as part of the Research Rotation course during the Spring 2009 semester. John worked on a variety of projects in both labs, including working to align the IR laser (part of Dr. R. Peebles' rapid-scan mid-IR spectrometer) and also assigning broadband scans (collected at the University of Virginia) for the CHClF2-HCCH complex. [Publications]
 Ashley A. (Osthoff) Elliott - Ashley was actually a graduate student working for Dr. Rebecca Peebles and did a lot of work writing LabView software to control various aspects of Dr. R. Peebles' rapid-scan IR spectrometer but we've included her here for her significant achievements in writing LabView software to simplify analysis of the data coming off the new CP-FTMW spectrometer. She wrote, from scratch, at least 3 separate LabView programs that analyzed the microwave data in different ways and she dealt (very good naturedly) with endless picky comments from both myself and Dan about her programs. Without Ashley's efforts, our spectral analysis would be significantly less efficient and a lot more painful. Ashley graduated from EIU at the end of 2009 with her Masters degree and is currently working for Abbott Laboratories in the Chicago area. [Publications]
Ashley A. (Osthoff) Elliott - Ashley was actually a graduate student working for Dr. Rebecca Peebles and did a lot of work writing LabView software to control various aspects of Dr. R. Peebles' rapid-scan IR spectrometer but we've included her here for her significant achievements in writing LabView software to simplify analysis of the data coming off the new CP-FTMW spectrometer. She wrote, from scratch, at least 3 separate LabView programs that analyzed the microwave data in different ways and she dealt (very good naturedly) with endless picky comments from both myself and Dan about her programs. Without Ashley's efforts, our spectral analysis would be significantly less efficient and a lot more painful. Ashley graduated from EIU at the end of 2009 with her Masters degree and is currently working for Abbott Laboratories in the Chicago area. [Publications]
 Patrick M. Lohan (Spring 2010) - Pat worked some on tweaking the LabView software written by Ashley and also on optimizing the discharge studies. His main contributions involved making additional measurements on the 29Si, 30Si and 13C isotopic species for 1-fluorosilacyclopentane. [Publications]
Patrick M. Lohan (Spring 2010) - Pat worked some on tweaking the LabView software written by Ashley and also on optimizing the discharge studies. His main contributions involved making additional measurements on the 29Si, 30Si and 13C isotopic species for 1-fluorosilacyclopentane. [Publications]
 Lena F. Elmuti (Summer 2010 - Summer 2013) - Lena worked initially on the assignment of the CH2ClF-HCCH spectrum from broadband scans carried out at the University of Virginia, as well as making measurements on the 35Cl and 37Cl isotopomers of the CH2ClF-H13C13CH species on the FTMW instrument at EIU; from this information Lena was able to determine the structure of this complex. Lena's recent projects have included more weakly bound complexes, and she became the expert in assigning the IR spectra that we collected at the Canadian LightSource synchrotron facility in May 2013, having assigned several thousand transitions in the spectrum of 1,1-dichloroethylene. Lena graduated with a BA in Chemistry and a BS in Biology in May 2013. After spending a few months in Palestine during Summer of 2013, she began at the University of Illinois Chicago Medical School in August 2013. [Publications]
Lena F. Elmuti (Summer 2010 - Summer 2013) - Lena worked initially on the assignment of the CH2ClF-HCCH spectrum from broadband scans carried out at the University of Virginia, as well as making measurements on the 35Cl and 37Cl isotopomers of the CH2ClF-H13C13CH species on the FTMW instrument at EIU; from this information Lena was able to determine the structure of this complex. Lena's recent projects have included more weakly bound complexes, and she became the expert in assigning the IR spectra that we collected at the Canadian LightSource synchrotron facility in May 2013, having assigned several thousand transitions in the spectrum of 1,1-dichloroethylene. Lena graduated with a BA in Chemistry and a BS in Biology in May 2013. After spending a few months in Palestine during Summer of 2013, she began at the University of Illinois Chicago Medical School in August 2013. [Publications]
 Brandon J. Bills (Summer 2010) - Brandon initially worked with Dan on the assignment of the CHBrF2-HCCH complex from a scan carried out at the University of Virginia before taking over the measurement of numerous isotopic species for the CHClF2-H2O complex, in particular being responsible for figuring out the deuterated spectra (CHClF2-D2O and CHClF2-DOH). Brandon also finally identified the tunneling doublets in the D2O species, allowing us then to locate the more widely spaced doublets in the parent isotopologue. [Publications]
Brandon J. Bills (Summer 2010) - Brandon initially worked with Dan on the assignment of the CHBrF2-HCCH complex from a scan carried out at the University of Virginia before taking over the measurement of numerous isotopic species for the CHClF2-H2O complex, in particular being responsible for figuring out the deuterated spectra (CHClF2-D2O and CHClF2-DOH). Brandon also finally identified the tunneling doublets in the D2O species, allowing us then to locate the more widely spaced doublets in the parent isotopologue. [Publications]
 Amelia J. Thomas (Summer 2010 - Summer 2012) - Amelia started work in the lab with some LabView programming and then on computing a potential energy surface (PES) for the tunneling motion of the H2O molecule in the CHClF2-H2O complex; at the end of the summer she assigned the 79Br isotopologue of the CHBrF2-H2O complex, with Dan assigning the 81Br isotopologue. Amelia has since extended the PES calculations to CHBrF2-H2O.
In Spring 2011 Amelia assigned the CH2F2...CO2 complex (which I looked at with Mike Serafin in about 2005 but we could not make any sense of the many lines). We returned to this complex in spring 2011 and made a scan with the new chirp instrument and were able to identify the internal motion that splits all the transitions into two. We think we now have a handle on this motion and have managed to assign several isotopic species as well as model the motion using ab initio calculations. Amelia was the first student through our new BS-MS program, defending her thesis in July 2012. [Publications]
Amelia J. Thomas (Summer 2010 - Summer 2012) - Amelia started work in the lab with some LabView programming and then on computing a potential energy surface (PES) for the tunneling motion of the H2O molecule in the CHClF2-H2O complex; at the end of the summer she assigned the 79Br isotopologue of the CHBrF2-H2O complex, with Dan assigning the 81Br isotopologue. Amelia has since extended the PES calculations to CHBrF2-H2O.
In Spring 2011 Amelia assigned the CH2F2...CO2 complex (which I looked at with Mike Serafin in about 2005 but we could not make any sense of the many lines). We returned to this complex in spring 2011 and made a scan with the new chirp instrument and were able to identify the internal motion that splits all the transitions into two. We think we now have a handle on this motion and have managed to assign several isotopic species as well as model the motion using ab initio calculations. Amelia was the first student through our new BS-MS program, defending her thesis in July 2012. [Publications]
 Don Jurkowski (Spring 2011) - Don (a pre-engineering student) worked on the CH2F2...HCCH complex (with Dan), our first complex scanned and entirely assigned on the chirp instrument. In addition to working on the initial assignment, Don worked on Stark effect measurements for this complex and some isotopically enriched species. [Publications]
Don Jurkowski (Spring 2011) - Don (a pre-engineering student) worked on the CH2F2...HCCH complex (with Dan), our first complex scanned and entirely assigned on the chirp instrument. In addition to working on the initial assignment, Don worked on Stark effect measurements for this complex and some isotopically enriched species. [Publications]
 Cori L. Christenholz (Summer 2011 - Summer 2013) - Cori worked in the summer and then stayed on and worked for Rebecca in the following semester. Cori originally assigned the complex between chlorofluoromethane and vinyl fluoride (while we were all in Columbus in June!) after earlier having assigned the difluoromethane...vinyl fluoride complex. Both complexes were scanned on our baby chirp instrument. After spending the Spring 2012 semester at Disney World in Florida, she returned to EIU and in May 2013 received her BS in Biology. After one more summer in the lab in Summer 2013 (where she was able to assign spectra for some new complexes, as well as making additional measurements on other projects), she returned to Disney World in August 2013 with plans to attend Physical Therapy school in the near future. [Publications]
Cori L. Christenholz (Summer 2011 - Summer 2013) - Cori worked in the summer and then stayed on and worked for Rebecca in the following semester. Cori originally assigned the complex between chlorofluoromethane and vinyl fluoride (while we were all in Columbus in June!) after earlier having assigned the difluoromethane...vinyl fluoride complex. Both complexes were scanned on our baby chirp instrument. After spending the Spring 2012 semester at Disney World in Florida, she returned to EIU and in May 2013 received her BS in Biology. After one more summer in the lab in Summer 2013 (where she was able to assign spectra for some new complexes, as well as making additional measurements on other projects), she returned to Disney World in August 2013 with plans to attend Physical Therapy school in the near future. [Publications]
 Yasser J. Dhahir (Summer 2012) - Yasser worked on assigning the complexes fluorobenzene-acetylene and chlorofluoromethane-propyne. [Publications]
Yasser J. Dhahir (Summer 2012) - Yasser worked on assigning the complexes fluorobenzene-acetylene and chlorofluoromethane-propyne. [Publications]
 Anthony A. Ernst (Summer 2012) - Anthony worked initially on measuring the dipole moment for the difluoromethane-carbon dioxide complex and then assigned the difluoromethane-propyne complex (including all the 13C isopotologues in natural abundance!) Anthony is now in the Agricultural Engineering program at UIUC. [Publications]
Anthony A. Ernst (Summer 2012) - Anthony worked initially on measuring the dipole moment for the difluoromethane-carbon dioxide complex and then assigned the difluoromethane-propyne complex (including all the 13C isopotologues in natural abundance!) Anthony is now in the Agricultural Engineering program at UIUC. [Publications]
 Nathan W. Ulrich (Fall 2012- Summer 2013) - Nathan assigned the rotational spectrum for the fluorobenzene...HCCH complex and all six 13C isotopolgues (in natural abundance), and determined the structure of the complex. He's also been working on the incorporation of the electron gun into the chirped-pulse instrument. A search for the benzene...HCCH complex also yielded a very nice spectrum, our first symmetric top assignment! Nathan graduated in Spring 2013, worked in the lab for the summer of 2013 and started a PhD at the University of Michigan in Ann Arbor in Fall of 2013. [Publications]
Nathan W. Ulrich (Fall 2012- Summer 2013) - Nathan assigned the rotational spectrum for the fluorobenzene...HCCH complex and all six 13C isotopolgues (in natural abundance), and determined the structure of the complex. He's also been working on the incorporation of the electron gun into the chirped-pulse instrument. A search for the benzene...HCCH complex also yielded a very nice spectrum, our first symmetric top assignment! Nathan graduated in Spring 2013, worked in the lab for the summer of 2013 and started a PhD at the University of Michigan in Ann Arbor in Fall of 2013. [Publications]
 Ashley M. Anderton (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) - Ashley joined the group as an undergraduate in Spring 2013 and will carry on in the Masters program as a graduate student beginning Summer 2013. She has so far worked on MP2 and DFT calculations of halobenzene complexes with acetylene and built a mixing controller to allow fine control of our sample mixtures. During Summer 2013 and Fall 2013 she has continued testing the effectiveness of some newer DFT methods at predicting the structures and properties of the complexes we study experimentally, and also assigned the spectrum of the complex between 1,1-difluorethylene and carbon dioxide and several of its isotopologues. After a successful MS defense in May 2014, Ashley continued a lot of work on the study of ionic and radical species in Summer 2014 before moving on to a job at Midwest Powder Coatings in the Chicago area. [Publications]
Ashley M. Anderton (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) - Ashley joined the group as an undergraduate in Spring 2013 and will carry on in the Masters program as a graduate student beginning Summer 2013. She has so far worked on MP2 and DFT calculations of halobenzene complexes with acetylene and built a mixing controller to allow fine control of our sample mixtures. During Summer 2013 and Fall 2013 she has continued testing the effectiveness of some newer DFT methods at predicting the structures and properties of the complexes we study experimentally, and also assigned the spectrum of the complex between 1,1-difluorethylene and carbon dioxide and several of its isotopologues. After a successful MS defense in May 2014, Ashley continued a lot of work on the study of ionic and radical species in Summer 2014 before moving on to a job at Midwest Powder Coatings in the Chicago area. [Publications]
 Anuradha G. Akmeemana (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) - Anu joined the group as a graduate student in Spring 2013 and worked initially on calculations for difluorobenzene dimer complexes with HCCH, and on identifying possible trimers in a mixture of fluorobenzene and HCCH. In Summer 2013 we received a scan of a difluorobenzene/acetylene mixture from the University of Virginia and after much painstaking analysis of the spectrum, Anu managed to find the spectrum of the 1,2-difluorobenzene...acetylene dimer, then later discovered a second spectrum that seems to indicate internal motion of some description (although attempts to fit this spectrum to our usual precision is proving to be quite a challenge and we have had to employ new programs (such as the ERHAM program of Peter Groner) to try to do this). She is currently working on improving her fits and trying to understand the tunneling motion. Anu defended her MS thesis in July 2014 and is currently back in Sri Lanka. [Publications]
Anuradha G. Akmeemana (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) - Anu joined the group as a graduate student in Spring 2013 and worked initially on calculations for difluorobenzene dimer complexes with HCCH, and on identifying possible trimers in a mixture of fluorobenzene and HCCH. In Summer 2013 we received a scan of a difluorobenzene/acetylene mixture from the University of Virginia and after much painstaking analysis of the spectrum, Anu managed to find the spectrum of the 1,2-difluorobenzene...acetylene dimer, then later discovered a second spectrum that seems to indicate internal motion of some description (although attempts to fit this spectrum to our usual precision is proving to be quite a challenge and we have had to employ new programs (such as the ERHAM program of Peter Groner) to try to do this). She is currently working on improving her fits and trying to understand the tunneling motion. Anu defended her MS thesis in July 2014 and is currently back in Sri Lanka. [Publications]
 Rachel E. Dorris (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) worked in the lab for 3 weeks during Spring 2013 as part of the CHM1440 Research Rotation course. She worked with Cori doing some experiments with the pulsed discharge nozzle and then measured the 13C isotopes for epoxybutane. So far Rachel has assigned spectra for the complexes vinyl fluoride...1,1-difluoroethylene and trifluoroethylene...carbon dioxide, as well as tidying up some measurements on some other complexes (such as additional isotopic measurements on vinyl fluoride...carbon dioxide). She is currently working on extending our experimental studies to include some more exotic radical and ionic species. [Publications]
Rachel E. Dorris (Spring 2013, Summer 2013, Fall 2013, Spring 2014, Summer 2014) worked in the lab for 3 weeks during Spring 2013 as part of the CHM1440 Research Rotation course. She worked with Cori doing some experiments with the pulsed discharge nozzle and then measured the 13C isotopes for epoxybutane. So far Rachel has assigned spectra for the complexes vinyl fluoride...1,1-difluoroethylene and trifluoroethylene...carbon dioxide, as well as tidying up some measurements on some other complexes (such as additional isotopic measurements on vinyl fluoride...carbon dioxide). She is currently working on extending our experimental studies to include some more exotic radical and ionic species. [Publications]
 Tabitha S. Songer (Spring 2013) also worked in the lab for 3 weeks during Spring 2013 as part of the CHM1440 Research Rotation course. Tabitha worked with Nate on measurements of the dipole moment of the fluorobenzene...HCCH complex. [Publications]
Tabitha S. Songer (Spring 2013) also worked in the lab for 3 weeks during Spring 2013 as part of the CHM1440 Research Rotation course. Tabitha worked with Nate on measurements of the dipole moment of the fluorobenzene...HCCH complex. [Publications]
 Kelly Jones (Spring 2014) worked in the lab for 3 weeks during Spring 2014 as part of the CHM1440 Research Rotation course. Kelly worked with Ashley on fine tuning the performance of the newly constructed mixing controller, which allowed us to better understand its performance in the generation of carbon chain radicals.
Kelly Jones (Spring 2014) worked in the lab for 3 weeks during Spring 2014 as part of the CHM1440 Research Rotation course. Kelly worked with Ashley on fine tuning the performance of the newly constructed mixing controller, which allowed us to better understand its performance in the generation of carbon chain radicals.
 Bailey Luce (Summer 2014) is working in the lab for Summer 2014, and has started initial work with Sarah (see below) on the measurement and assignment of the cyclopropylmethyldifluorosilane molecule sent to us by Dr. Gamil Guirgis. Bailey went on to measure the dipole moment of this molecule and is currently measuring additional isotopic species for the benzene...acetylene complex in the hope that we may eventually be able to derive an equilibrium structure for this dimer.
Bailey Luce (Summer 2014) is working in the lab for Summer 2014, and has started initial work with Sarah (see below) on the measurement and assignment of the cyclopropylmethyldifluorosilane molecule sent to us by Dr. Gamil Guirgis. Bailey went on to measure the dipole moment of this molecule and is currently measuring additional isotopic species for the benzene...acetylene complex in the hope that we may eventually be able to derive an equilibrium structure for this dimer.
 Sarah Stettner (Summer 2014) is also working in the lab for Summer 2014. Her introduction to all things spectroscopic was provided by assignment of spectra for the molecule cyclopropylmethyldifluorosilane (sample courtesy of Dr. Gamil Guirgus from the College of Charleston, South Carolina). Sarah then moved on to become the lab's expert on the ERHAM internal rotation fitting program of Dr. Peter Groner. Currently she is making great progress in learning how to apply the BELGI program of Dr. Isabelle Kleiner to some of our previously assigned spectra.
Sarah Stettner (Summer 2014) is also working in the lab for Summer 2014. Her introduction to all things spectroscopic was provided by assignment of spectra for the molecule cyclopropylmethyldifluorosilane (sample courtesy of Dr. Gamil Guirgus from the College of Charleston, South Carolina). Sarah then moved on to become the lab's expert on the ERHAM internal rotation fitting program of Dr. Peter Groner. Currently she is making great progress in learning how to apply the BELGI program of Dr. Isabelle Kleiner to some of our previously assigned spectra.
 Erica Webster (Fall 2014) worked in our lab in Fall of 2014. Erica worked initially on synthesis of DCCD and the measurement of the benzene...DCCD isotopic spectrum before moving on to try to assign the spectrum of a molecule supplied by Prof. Gamil Guirgis. However, there were issues with signal intensity and sample purity and so she is currently cataloging the very dense spectrum of the gauche conformer of 1,2-dichloroethane. We hope to use as a precursor in some future pulsed discharge nozzle experiments (hence we want to know where all the monomer lines are).
Erica Webster (Fall 2014) worked in our lab in Fall of 2014. Erica worked initially on synthesis of DCCD and the measurement of the benzene...DCCD isotopic spectrum before moving on to try to assign the spectrum of a molecule supplied by Prof. Gamil Guirgis. However, there were issues with signal intensity and sample purity and so she is currently cataloging the very dense spectrum of the gauche conformer of 1,2-dichloroethane. We hope to use as a precursor in some future pulsed discharge nozzle experiments (hence we want to know where all the monomer lines are).
 Rebecca D. Nelson (Spring 2015) worked in the lab for 3 weeks as part of the CHM1440 Research Rotation course. In that short time Becky managed to assign the rotational spectrum for the 1,2-difluorobenzene...DCCD isotopologue.
Rebecca D. Nelson (Spring 2015) worked in the lab for 3 weeks as part of the CHM1440 Research Rotation course. In that short time Becky managed to assign the rotational spectrum for the 1,2-difluorobenzene...DCCD isotopologue.
 Mikayla L. Grant (Spring 2015, Summer 2015) worked in the lab for 3 weeks as part of the CHM1440 Research Rotation course and also did some research in the Summer of 2015. Mikayla worked extensively on some MathCad routines to assist in our data analysis procedure.
Mikayla L. Grant (Spring 2015, Summer 2015) worked in the lab for 3 weeks as part of the CHM1440 Research Rotation course and also did some research in the Summer of 2015. Mikayla worked extensively on some MathCad routines to assist in our data analysis procedure.
 Asela "Samee" Dikkumbura (Spring 2015, Summer 2015, Fall 2015) started work in the lab as a graduate student of Dr. Rebecca Peebles. Samee has been working on assignment of the complicated and very dense hyperfine patterns that arise from the presence of 1 or 2 Cl atoms in some of the molecules that we are using as potential precursors for preparation of gas-phase cations.
Asela "Samee" Dikkumbura (Spring 2015, Summer 2015, Fall 2015) started work in the lab as a graduate student of Dr. Rebecca Peebles. Samee has been working on assignment of the complicated and very dense hyperfine patterns that arise from the presence of 1 or 2 Cl atoms in some of the molecules that we are using as potential precursors for preparation of gas-phase cations.
 Seri P. Kamari (Spring 2015, Summer 2015, Fall 2015) also started work in the lab as a graduate student of Dr. Rebecca Peebles. Seri began work in the lab on the analysis of some Far IR spectra collected at the Canadian Light Source synchrotron facility. More recently she began work on the calculation of potential energy surfaces for a series of neon complexes of fluorinated benzenes in order to assist us with the assignment of the 1,2-difluorobenzene...Ne complex and has been working out how we can utilize the Atoms in Molecules (AIM) model to gain additional insight into our experimental studies.
Seri P. Kamari (Spring 2015, Summer 2015, Fall 2015) also started work in the lab as a graduate student of Dr. Rebecca Peebles. Seri began work in the lab on the analysis of some Far IR spectra collected at the Canadian Light Source synchrotron facility. More recently she began work on the calculation of potential energy surfaces for a series of neon complexes of fluorinated benzenes in order to assist us with the assignment of the 1,2-difluorobenzene...Ne complex and has been working out how we can utilize the Atoms in Molecules (AIM) model to gain additional insight into our experimental studies.
 Emily N. Pinter (Fall 2015) worked on assignment of the rotational spectrum and dipole moment measurements for perfluoropropene. Measurements on both the chirped-pulse FTMW and the resonant cavity instrument revealed many more transitions than had been previously characterized for this molecule. Also, our higher resolution measurements showed no indication of internal rotation splittings from the CF3 group. This work will be useful for our future work where we intend to use this molecule as a precursor in our pulsed discharge nozzle.
Emily N. Pinter (Fall 2015) worked on assignment of the rotational spectrum and dipole moment measurements for perfluoropropene. Measurements on both the chirped-pulse FTMW and the resonant cavity instrument revealed many more transitions than had been previously characterized for this molecule. Also, our higher resolution measurements showed no indication of internal rotation splittings from the CF3 group. This work will be useful for our future work where we intend to use this molecule as a precursor in our pulsed discharge nozzle.
 Bill C. Trendell (Spring 2016) joined our masters program in Fall 2015 and started work in our lab in Spring 2016. He is working on implementation of the QTAIM (Quantum Theory of Atoms in Molecules) model to a series of carbon dioxide complexes so that we may better understand the interactions that guide solvation of molecules by supercritical carbon dioxide. Future plans include application of the SAPT (Symmetry Adapted Perturbation Theory) and Natural Bond Order (NBO) approaches to these and other complexes to gain further insight into the strength and nature of the interactions in such weakly bound clusters.
Bill C. Trendell (Spring 2016) joined our masters program in Fall 2015 and started work in our lab in Spring 2016. He is working on implementation of the QTAIM (Quantum Theory of Atoms in Molecules) model to a series of carbon dioxide complexes so that we may better understand the interactions that guide solvation of molecules by supercritical carbon dioxide. Future plans include application of the SAPT (Symmetry Adapted Perturbation Theory) and Natural Bond Order (NBO) approaches to these and other complexes to gain further insight into the strength and nature of the interactions in such weakly bound clusters.
Publications and presentations for each student are available through the [Publications] link after each name (provided by Google Scholar).
Pictures, data, and other items posted on this web page are for personal use only, and any other uses require permission of the PI or co-PI.
References
T.J. Balle and W.H. Flygare, Rev. Sci. Instrumen., 52, (1981), 33. Back to text
Page maintained by sapeebles (add "@eiu.edu" to form email address)Go Back to S.A. Peebles' home page.