

Ch 19, Temperature Absolute Temperature Scale
Constant-volume gas thermometer: When the column of mercury is adjusted so the top of the mercury is at the "0" mark on the scale, the volume of the gas is a constant. The height of the mercury column, h, then measures the pressure of the gas. This pressure can be used as a measure of temperature. (The pressure of the gas is a thermometric property).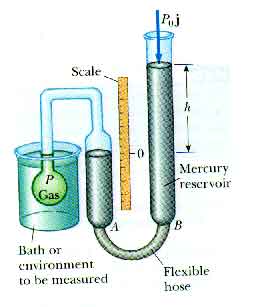
Such a constant-volume gas thermometer gives easily reproducible results over a wide range of temperatures. It is accurate over a wide range of conditions -- as long as we avoid getting close to the condensation temperature of the gas. It is interesting to extrapolate this graph to see where the pressure would go to zero.
If we use constant-volume gas thermometers filled with gasses of different kinds or at different pressures, we will still measure the same temperatures. That is why a constant-volume gas thermometer is useful! And an extrapolation of each of their graphs to zero pressure occurs at the same temperature, - 273.15oC.
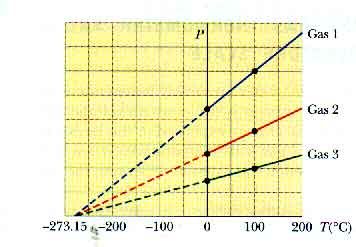
We can -- and will -- use this to create a new temperature scale, the absolute temperature scale. This common temperature at which all the constant-volume gas thermometers converge is called absolute zero. The size of the units on this scale are the same as on the Celsius temperature scale. The units are called kelvins and are indicated by K -- without a "degree" sign. This is also known as the Kelvin temperature scale.
(c) Doug Davis, 2002; all rights reserved
Return to Temperature ToC