

Ch20; Heat and the First Law of Thermodynamics
Heat Capacity The heat capacity C depends upon the material and the amount of the material
Q = C orT
C = Q / That is, if heat Q is necessary to change the temperature of an object by an amountT
T, then the heat capacity C of that object is given by
C = Q / The energy required to change the temperature of an object depends upon the material of the object and upon the mass of the object. We can take care of the mass m of the object explicitly and write this asT
Q = c m
T
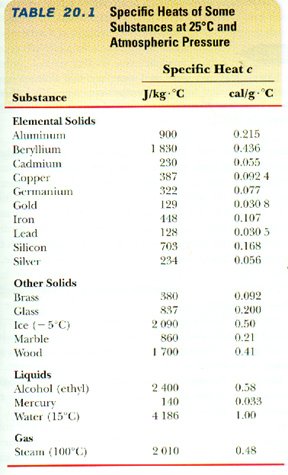
where this lower case c is the specific heat of the material. That is,
c = Q / m
T
That is, c, the specific heat of a material is the ratio of the amount of heat Q necessary to change its temperature by an amountT divided by that temperature change and the mass m of that material.
Conservation of Energy
As one part of a system gains energy another part of the system looses energy. We can write that as
Qgain = - Qloss or
Qcold = - Qhot or
Qgain + Qloss = 0 or
Qcold + Qhot = 0 All of those equations simply mean that no heat is added to or taken from "the system". Energy is transferred from one part of the system to another.
Return to Ch20 ToC (c) Doug Davis, 2002; all rights reserved