

Ch20; Heat and the First Law of Thermodynamics
Heat and Internal Energy Internal energy is the energy of the atoms and molecules -- translational energy, rotational energy, and vibrational energy.
Heat is internal energy that is exchanged between two objects due to their difference in temperature.
Heat always transfers, or flows, from a body at higher temperature to one at lower temperature.
Heat and internal energy are obviously connected or associated; but they are not the same thing. Heat is a transfer of internal energy due to a difference in temperature. This is analogous to the connection between work and energy. When you do work on a system you change its energy. Work is a transfer of energy.
UNITS
One calorie, cal, is the amount of heat necessary to raise the temperature of 1.0 g of water from 14.5oC to 15.5oC
Notice that one "food calorie", 1 Cal, is one thousand "heat calories"; that is,
1 Cal = 1000 cal = 1 kcal
One British thermal unit, BTU or btu, is the amount of heat necessary to raise the temperature of 1.0 pound (lb) of water from 63oF to 64oF
The Mechanical Equivalent of Heat
Work and energy are related. Work can raise the temperature of a system.
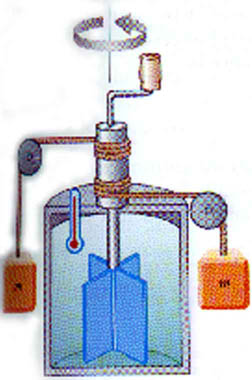
Joule -- after whom the SI unit of energy is named -- did early experiments showing the connection between heat and work. He found that direct connection to be
1 cal = 4.186 J
This is known as the Mechanical Equivalent of Heat. You will do a lab experiment later this semester in which you measure this for yourself.
Return to Ch20 ToC (c) Doug Davis, 2002; all rights reserved